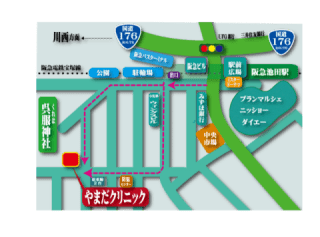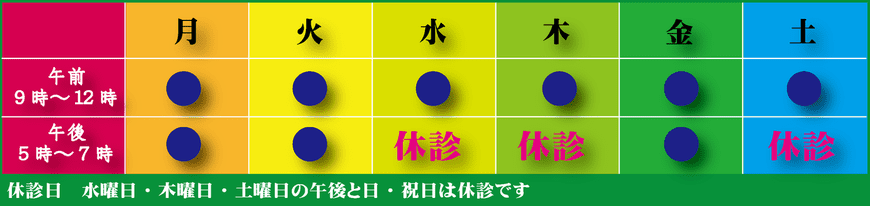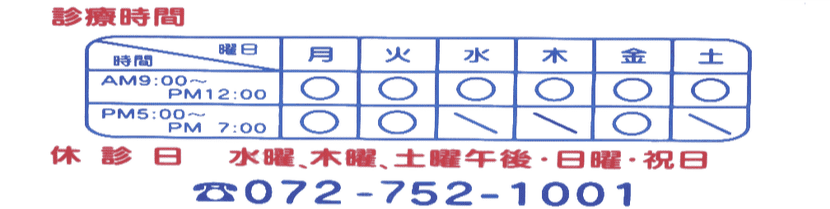
発熱など風邪様症状の患者様へ |
※受診される前に必ずお電話072-752-1001を下さい。 受診時間などをご案内いたします。 窓越しに検査をしてから院内に入って頂きますのでご了解お願い致します。 ※下記の風邪様症状事前問診票の記入・送信をお願い致します。 |
風邪様症状の方は電話連絡の後で下記の事前問診票を送信してください
2023-05-16
やまだクリニックからのお知らせ
2024-03-31
2024年からの新型コロナワクチン予約終了
2023-11-23
2023年秋開始新型コロナワクチン接種は今年は終了致します
2023-12-19
2023年インフルエンザ予防接種は終了致しました
2023-09-14
2023年秋開始XBB株対応新型コロナワクチン予約
2023-08-10
| RSS(別ウィンドウで開きます) | もっと見る |
デジタルカタログ

保険外診療料金表

健康診断問診票

インフル予防接種問診票